pH Measurement Comparisons
Test strips vs pH meter
This
page compares the use of commercial
pH strips against a calibrated pH meter. The data were collected on
16th October, 7th November, 13th November and 10th December
2011 on various ciders and juices which I was pressing in the 2011 season.
pH strips
- Merck
(branded BDH) strips aka 'Colorphast'. Catalogue 31501. These strips probably > 10 years
old. Opaque plastic bonded strips - methyl orange is the likely indicator.
Colour comparison printed on box (red to orange) for pH values 2.5,
3.0, 3.3, 3.6, 3.9, 4.2, 4.5 (3.3 and 3.6 almost identical)
Viewed outdoors in cloudy-midday conditions.
- Vinoferm
strips. Catalogue 013.073.2 Freshly purchased. Translucent paper
strips - bromophenol blue is the probable indicator. Colour comparison
printed on strips (yellow to purple) for pH values 2.8, 3.1, 3.4, 3.6,
3.8, 4.0, 4.3, 4.6 Viewed outdoors in cloudy midday conditions via transmitted and reflected light.
- Lyphan strips. Catalogue L 656-8. These strips probably ca 8 years old. Translucent paper strips - bromophenol blue is the probable indicator.
Colour comparison printed on strips (yellow to purple) for pH values
3.0, 3.2, 3.4, 3.6, 3.8, 4.0, 4.2, 4.4 Viewed outdoors in cloudy midday conditions via transmitted and reflected light.
All strip values were interpolated where required, if falling between 2 calibration points.pH meter
- Hanna pHep5 HI-98128 dipstick pH meter calibrated at pH 4.01. Quoted resolution ± 0.01 pH. Quoted accuracy ± 0.05 pH.
Results and Discussion
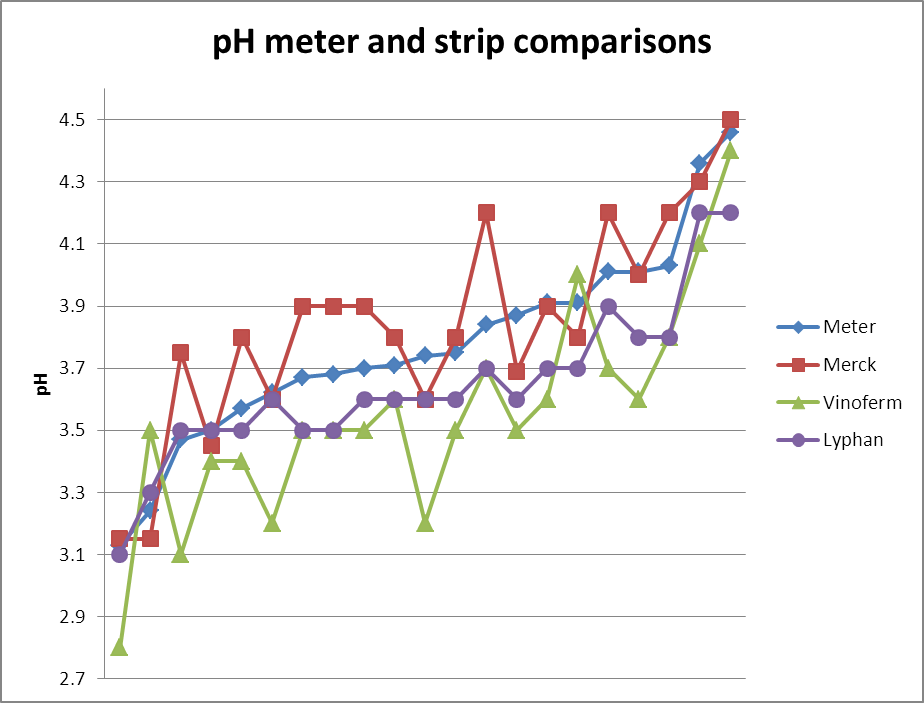
The graph plots 20 data points covering a typical pH range for UK cider apples. The data show that the Merck strips seem
to read higher than the pH meter over much of their range, while the
Vinoferm strips typically read lower. The Lyphan strips seem to track the pH
meter reading best, but even so are typically lower. The Vinoferm indicator bar is poorly laid down on
the paper and gives a blotchy appearance when in use, rather than a
continuous colour. Lyphan is better in that respect. It is quite hard
to read both Lyphan and Vinoferm strips - in the middle part of their
range the indicator bar does not seem to correspond well in hue with
the printed reference strips, effectively being shades of grey. This is more noticeable on the Vinoferm strips. I
was also concerned that the Lyphan and Vinoferm strips do not respond correctly to
the pH 4 standard buffer used to calibrate the pH meter, both coming in
nearer to pH 3.8 than pH 4 (but still in shades of poorly-matching
grey). The Merck strips by contrast give the correct reading with the
pH 4 buffer. (That data point is not shown on the graph because it is not juice nor cider, which is the topic of interest).
Claude
Jolicoeur did some stats on my data for Root Mean Square (RMS) error,
Bias and Standard Deviation (SD) and and reported as follows:
For Merck - RMS error 0.16 is not too bad,
bias is lowest at .07
SD at 0.15, very close to RMS error - no much improvement if we
correct readings with bias
For Vinoferm - RMS error is the greatest, close to 0.3 units of pH
bias is -0.22, indicating the avg reading is 0.22 pH too low -
this is quite large and should be corrected, i.e. one should add 0.22 to
the Vinoferm reading to assess the correct pH
SD 0.18 is RMS error once readings are corrected by the bias
value - this is more reasonable, but still the highest.
Lyphan, same RMS error as Merck, but with a larger bias. So this one is
more easily corrected: one should add 0.13 to the strip reading to get a
more accurate pH value. The SD indicates that the RMS error would then
become 0.09 - less than 0.1 unit of pH, which would be quite good.
I would prefer to buy Lyphan strips, but Merck comes close... Vinoferm
is definitely the least accurate.
This
is the first time I have done such a formal comparison and frankly I am
disappointed by the performance of all the pH strips. I think there are two problems:
1. The inherent limits of the physics and chemistry. Acid/base
indicators are complex organic dyes which change colour due to changes
in electron orbital energy levels depending on whether they are
protonated or not. At either end of the range the changes are easy to
spot e.g. bromophenol blue goes from yellow when fully protonated at pH
< 3 to blue at pH > 4.5 when it is not. But the intermediate hues of
partial protonation are effectively shades of grey and so it is very
difficult to assign a specific pH to each intermediate shade with any
accuracy. I suspect (but I do not know) that this might alter depending
on what else is in the solution too and probably affects the exact way
in which the dye is ionising.
2. The printing process or the QC around the reference colours. These
are presumably printed on the strips as normal permanent (and pH
independent) dyes. The care with which these are selected and QC checked
batch to batch probably varies. I expect a branded lab quality strip
will be better in this regard than just something slung together that's
"good enough for home winemakers". I think the data and Claude's
analysis bears this out. Claude has suggested a correction offset figure
for the strips but I wonder if this would be consistent from batch to
batch if the manufacturing tolerances are poor.
Conclusions
If you
can afford to buy and care for a pH meter then do that, bearing in mind
that a meter
is more costly, needs care in use and storage, and must be
calibrated for every session. But even a bottom of the range dipstick-type branded meter from a reputable manufacturer (eg Hanna UK or Hanna USA)
will nowadays offer an accuracy of ± 0.1 pH units and may have
replaceable or renewable electrodes. You also need calibration buffer
(pH 4 at minimum), cleaning and storage solutions if it is to serve you
well. If you can get hold of good
quality branded lab pH strips then they are passable substitutes but
with poorer accuracy . The cheapo hobby winemaking pH strips are less
good but probably any measurement is a good deal better than
nothing at all! Even a rough idea of pH will still be
helpful in deciding the level of SO2 to add, which is also subject to many approximations in itself!
Back to my pH and acid page
Last updated 19.12.2011