Sulphur
Dioxide - the Cidermaker's Friend
Sulphur dioxide or SO2 is the cidermaker's
friend. It has been used for hundreds of years (and possibly thousands for winemaking).
A quote I love to use is from John Beale, a contributor to
Evelyn's Pomona of 1664. He wrote:
“Lay
brimstone on a rag, and by a wire let it
down into the cider vessel, and there fire it; and when the vessel is
full of
the smoak, the liquor speedily pour’d in, ferments the
better”
In simple
terms what
happens is that the sulphur dioxide obtained from burning
sulphur (brimstone) inhibits or kills most spoilage yeasts, moulds and bacteria,
while
permitting the most desirable fermenting yeasts (such as Saccharomyces
cerevisiae or uvarum) to multiply and to
dominate the conversion to
alcohol. Only small amounts of sulphur dioxide are used, and its
effectiveness
depends quite critically on the pH of the juice (see below). Cider
juice,
when pressed, contains large numbers of yeast, mould and bacteria which
we do
not want and only a very few of those that we do. The sulphite levels
in the
table have been empirically established based on laboratory
investigations of
the sensitivity of these cider micro-organisms and the likely amounts
of
natural ‘sulphite binding’ substances in the juice,
in order to establish the
dominance of benign fermenting yeasts and hence a much reduced chance
of taints
and off-flavours in the finished product.
Sulphite is quite natural, occurring in the atmosphere in the gaseous
form of sulphur dioxide (SO2) as a result of volcanic processes, and in
the human body as a result of the breakdown of sulphur-containing
proteins during digestion. Many yeasts also produce it during
fermentation, reducing naturally occurring sulphate to sulphite. Of
course it can be toxic, even lethal, in the wrong dose and in the wrong
place, but so also can common salt and water.
The
form of sulphite used in cider or winemaking nowadays is as the
"Campden tablet",
or as sodium or potassium metabisulphite powder (which should not
be breathed in as it is irritant). In acid solutions such as
apple juice these materials liberate sulphur dioxide directly into the
liquid. (You can burn 'sulphur string' or 'suphur candles' in barrels
as Dr
Beale did, but it is impossible to be accurate with the sulphite dose
then). The amounts used are tiny, less than 200 parts per million
(ppm), and most of it gets quite rapidly bound up in the cider and
is no longer 'free'. Note that a very few people, principally
asthmatics, are
hypersenitive to sulphur dioxide in the free state which is why it is
labelled as a food allergen. Once bound in a beverage, it is no
longer a problem. The best documented cases of sulphite
sensitivity were from its use (now banned) at high levels on pre-packed
salads or pre-cut potatoes, where the high free SO2
levels could be a severe problem for asthmatics. The data on wine is
much more equivocal and where people have been challenge tested in
clinical trials far fewer of them prove to be sulphite sensitive than
they imagine. There are many other things in fermented beverages which
can cause adverse reactions, in addition to sulphite.
So.......how
much sulphite should I
add?
The effectiveness of sulphite depends very much on the juice pH. The Table below gives an approximate idea of how much sulphite needs to be
added to
a juice at a given pH before fermentation, depending on whether you
intend to
add a cultured yeast or allow the wild yeasts to do the job.
In
both
cases, addition of sulphite is important to kill bacteria, moulds
and adverse wild yeasts, while allowing the beneficial ones to
flourish. If a cultured yeast is added, sulphite addition
should
be made
12- 24 hrs before the addition of the yeast, or the added yeast will be
severely inhibited. Added cultured yeasts will start
within a day or two. If you are allowing wild yeast to build
up and to start the fermentation, you can use the 'total yeast
kill' dose in the left hand column, but the beneficial yeasts that
survive this may take several weeks to build up and get going. Things
will move quicker if you use the 'partial yeast kill' column, which
allows more wild organisms such as 'apiculate' yeasts to survive and will
give a different flavour balance. The dose is given both in parts per
million
of
sulphur dioxide (ppm or milligrams per litre) and the equivalent in
Campden
tablets per gallon (which are formulated so one tablet gives per gallon
gives 50 ppm). For more about the origin of Campden tablets click here.
It's
often
easier to use a stock solution of sulphur dioxide. To make a 5% stock
solution, dissolve around
10 grams
of sodium or potassium metabisulphite in 100 ml of water. (The
metabisulphite salts contain around 50 - 60% of available SO2
depending
on how they've been stored). Then 1 ml of this per
litre
of juice (5 ml per gallon) corresponds to 50 ppm (parts per million) of
SO2.
Note
that the correlation between titratable acid (TA) and pH is only
approximate
(as the graphs elsewhere make quite clear!). pH is the best measurement.
Sulphite Addition Table
|
pH
|
Approx TA
(% malic)
|
For
total yeast kill
(when adding cultured yeast)
|
For partial yeast kill
(for wild yeast fermentation)
|
|
SO2
(ppm)
|
Campden tablets
per gallon
|
SO2
(ppm)
|
Campden tablets
per gallon
|
|
3.0
– 3.3
|
1.2
– 0.8
|
50
|
1
|
nil
|
nil
|
|
3.3
– 3.5
|
0.8
– 0.6
|
100
|
2
|
50
|
1
|
|
3.5
– 3.8
|
0.6
– 0.3
|
150
|
3
|
100
|
2
|
|
>
3.8
|
<
0.3
|
add more acid!
|
add more acid!
|
150
|
3
|
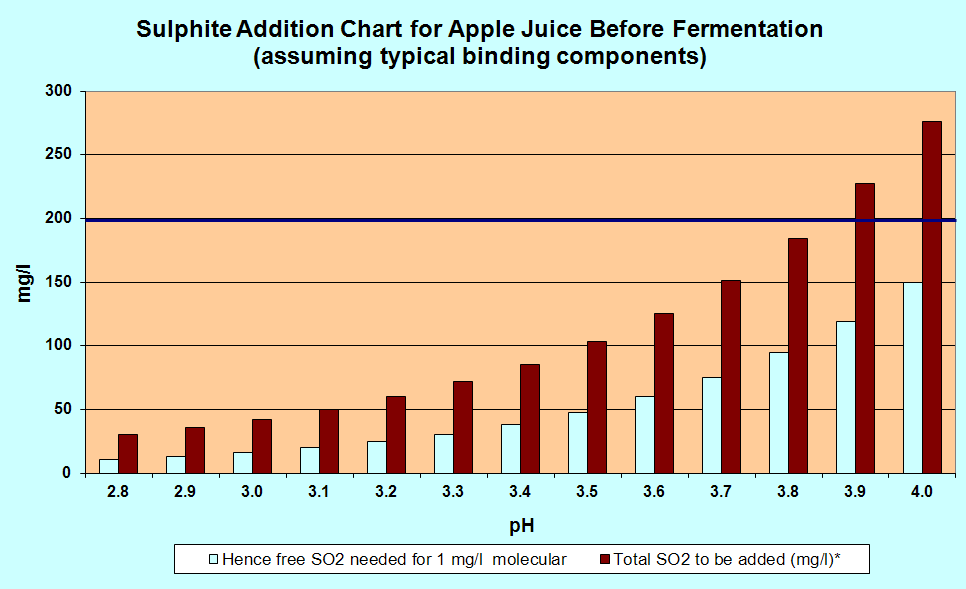
A more accurate chart
The
chart below gives the same sort of information, but is for those who
are measuring the pH accurately and dispensing their SO2 by volume from a stock solution and not by Campden tablets. The red bars give the amount of SO2 you need for a more or less total kill of the wild yeasts. If you want a partial kill, use only half the amount.
Addition of sulphite after fermentation
The table and chart above is
for use of sulphite as an antimicrobial before fermentation. Sometimes it is also used after
fermentation has all finished, at
racking, storage and bottling. The reason for this is partly
antimicrobial but also because it acts as an antioxidant. Or, rather,
it mops up the initial products of oxidation such as hydrogen peroxide
and aldehydes, preventing them going on to give sherry-like or
'oxidised' off-flavours. In those cases it is usual to add a
fixed amount of 50 ppm each time (up to the total legal limit of 200
ppm when all additions are summed together) with a view to achieving a
residual 30 ppm of free SO2 the next day. This is because the
antioxidant properties of sulphur dioxide are not affected by pH.
If you are planning to pasteurise a back-sweetened cider to stop it re-fermenting, the addition of 50 ppm SO2
at bottling also offers the benefit of blocking the Maillard reaction
between amino acids and sugars, which helps to minimse the development
of "cooked" flavours from the pasteurisation process.
How does sulphite work so selectively against adverse micro-organisms?
It's an interesting question - why does it act more effectively on the
'undesirable' yeasts and other microbes? The answer is not clear but it
seems that microbial sensitivity to sulphite is the norm and resistance
is a mutation. Most natural weak acid preservatives (eg vinegar (acetic
acid), benzoic and and sorbic acids) are believed to work by being able
to enter into microbial cells by molecular diffusion through the cell
membranes because they are in part lipophilic (fat soluble). Once
inside, they ionise and increase the acidity (lower the pH) and the
cell homoeostasis mechanism has to work very hard to pump out protons
to restore the pH. Eventually the cells become so exhausted that they
run out of ATP (their energy source) to do this and give up and die or
at least stop growing. Sulphite is believed to work in the same way as
other weak acid preservatives. In addition, it can bind to or disrupt
sulphur bridges in cell proteins, inhibit enzymes, and interfere with
DNA replication.
Sulphite-resistant organisms appear to have the ability to synthesise
acetaldehyde in response, more readily than other microbes. This binds
up the sulphite and makes it inactive hence it is neutralised and the
cell survives and continues to grow. It cannot be an accident that
acetaldehyde is a key intermediate (the last step in the chain) of the
synthesis of alcohol from glucose. In other words, the same mutation
which confers the ability to be a wine or cider yeast (smooth
fermentation of sugar to relatively high alcohol levels) entails the
facile ability to generate higher levels of acetaldehyde than normal,
which will bind up the sulphite and make it inactive. This is the
working hypothesis that most fermentation microbiologists go with.
More information for tecchies!
All the dosage information above is derived from long-standing work at the Long
Ashton Research Station and other wine research institutes, which
started in the 1950's and culminated in
the late 1970's. It is based on the empirical fact that the
level
of molecular SO2
required to kill adverse yeasts and bacteria but to allow
beneficial ones to flourish is around 1 part per million. To
get
this level of molecular SO2 you actually need a
lot more free SO2
because there is
a pH related equilibrium which keeps most of the SO2
in the
inactive bisulphite ion form. Hence, in the table and chart above, the amount of SO2
you need to add depends on the pH.
Unfortunately, that's not all the story. When you add SO2
to
juice or cider, some of it becomes bound
to juice components like glucose, galacturonic acid,
pyruvate
etc. Hence the total SO2 you need to add must
also take
account of this binding. It is the total
SO2
which is given in the
table and chart above. This is not an exact science because it needs to make
certain assumptions about the levels of the binding components, which
will differ depending on the nature of the fruit, how many rotten
apples got in etc etc! So the figures given in the table and chart are
necessarily approximate.
In the table, the column for total yeast kill is based on a target
value
of 1 ppm molecular SO2 and for partial yeast
kill is based
on 0.5 ppm.
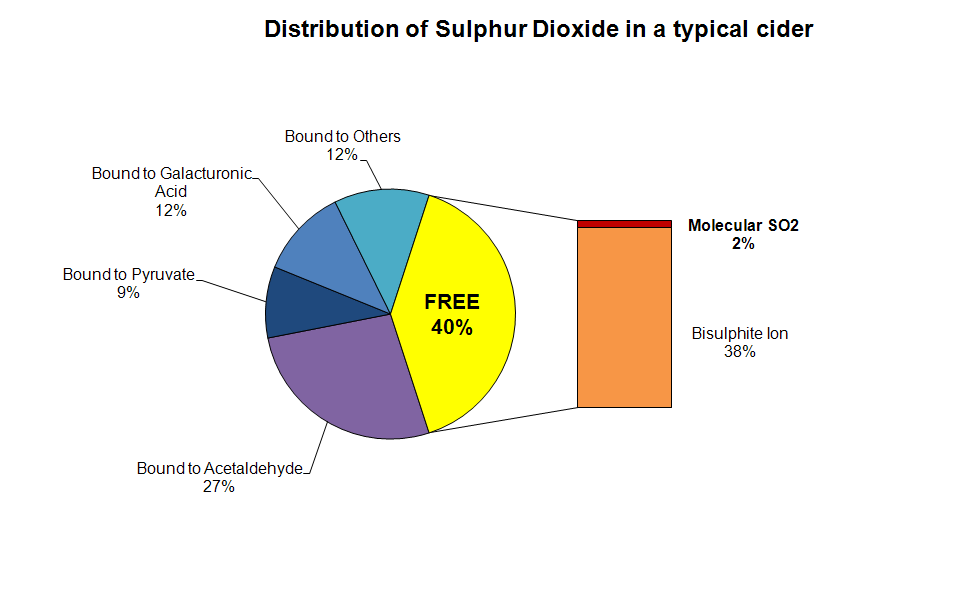
Here's a summary which depicts the various forms in which SO2
might exist in a fermented cider after sulphite addition. The same general principles apply to a juice
before fermentation, though the binders are different. The most
important point is that only the small amount of molecular SO2 is actually effective as an anti-microbial.
If you want to know more, and to find out how that distribution is calculated, and you have software which can read Excel
spreadsheets, then download this file on
sulphite
binding and addition.
It contains three worksheets. The first one gives a table of
molecular, free and total SO2 at different pH
values (and is
more comprehensive than the table above). The second is just a
graphical representation of the free and total SO2
columns
(for a 'typical' apple juice) and is where the accurate addition chart comes from. The third worksheet gives more detail on
how the binding calculation is carried out. There are also
some
literature references given to show where the science comes
from
(and my publications page
also contains a
couple of downloads of relevant papers which should give some
further background, although they are focussed more on the addition of
SO2 to cider after fermentation than to juice
beforehand ).
Last edited 7th July 2011
Back
to the Contents
Page